Extending the role of fibers for face masks manufacturing: Ahlstrom Reliance® SMS and Dextex wetlaid nonwoven receive validation from the French Government
As the COVID-19 outbreak continues to expand globally, the supply chain for face masks continues to be stressed with demand exceeding available supplies. Ahlstrom- has been actively working on alternative technologies and capabilities expansions, with synergies among its sites and new dedicated production lines. Now the company, and the Medical Business Unit specifically, are taking another step forward, going beyond the scope of the general medical offer.
The French National Agency for the Safety of Medicines and Health Products (ANSM) has recently issued guidance to provide policies that help expand the availability of face masks for non-sanitary use, which now include two new categories: “Individual filter masks for use by professionals in contact with the public (Category 1)” and “Collective protection filter masks to protect an entire group (without contact with the public)”.
In accordance to the newly implemented guidelines, our Reliance® SMS 200, Reliance® SMS 300, Reliance® Dextex 200 and Reliance® Dextex 300 technologies received the validation by ANSM as “face mask Category 1”, in the case of Reliance® Dextex 200 and Reliance® 300 and “surgical face mask” for Reliance® SMS 200 and Reliance® SMS 300.
To accomplish this, Jonathan Laloum, Global Product Manager SBS, conducted an extensive work in partnership with SF2S (Société Française des Sciences de la Stérilisation), notably the highest scientific source for the sterilization industry in France, and the Direction Générale de l’Armement (DGA), the French Government Defense procurement and technology agency.
The tests conducted by the DGA confirmed the performance compatibility of our Reliance® SMS material with surgical face masks types thanks to its excellent breathability and bacterial filtration efficiency whereas Reliance® Dextex proved to ensure permeability to air and to perform in protection efficiency at a level compatible to the category 1 face masks currently in use.
Reliance® SMS 200, Reliance® SMS 300, Reliance® Dextex 200 and Reliance® Dextex 300, manufactured at the Medical business plants in Windsor Locks, United States and Mundra, India, and converted at the site in Pont Audemer, France, have already been supplied to hospitals across France, to the French Government and distributors.
“I am very proud to see that in the face of an extremely challenging environment my team is looking above and beyond the scope of our existing product offer and challenges our product portfolio specifications to meet the new demand in facemask in France and worldwide. Beyond innovation this crisis calls for creativity to allow for more solutions to come to life,” says Lionel Bonte, Vice President Medical Business Unit at Ahlstrom.
End-user education: our support in helping hospital staff to create face masks
The cooperation between the Medical business and SF2S was not limited to the validation of the material. Thanks to Dr. Christophe Lambert and his team, Chambery hospital developed and designed guidelines instructing end-users on how to create a face mask using a pre-cut sheet of the Reliance® material.
The process is easily explained in a step-by-step video: the SMS and wetlaid sheets can in fact be turned into a full-functioning face mask in a few simple steps using a sewing machine. Pre-sheeted material has already been supplied to many healthcare facilities across France from the Medical plant in Pont Audemer. The rapid response to this new, growing demand was also made possible thanks to the new state-of-the-art converting and packaging line recently activated at the French site.
What’s next: new opportunities in the COVID-19 pandemic fight and beyond
In light of the recent events mentioned above, and thanks to the long-term experience of the Medical business in face mask fabrics manufacturing, the use of the Reliance® SMS and Reliance® Dextex materials will have additional opportunities. Reliance® Dextex is already qualified for face mask applications at the Windsor Locks site, United States, and qualification will also be obtained shortly at the facility in Brignoud, France and Malmedy, Belgium.
Moreover, the team has been actively engaging with Fortune 500 companies in the United States for the commercialization of the face mask fabrics manufactured at the Windsor Locks plant.
All these initiatives ultimately aim at expanding the role of our fiber-based materials for the manufacturing of face masks, now when it’s most needed as well as in the longer term.
About Reliance® SMS and Reliance® Dextex
Reliance® SMS and Reliance® Dextex wetlaid nonwovens are extensively used by the Medical business in the manufacturing of sterilization wraps for sterile barrier system. Reliance® wraps play a key role in contributing to patient’s safety and preventing hospital acquired infection. They help to prevent microorganisms, such as bacteria, viruses and spores, from easily gaining access to the tray content, allow sterilization of surgical instruments, provide physical protection, maintain sterility up to the point of use and allow aseptic presentation.
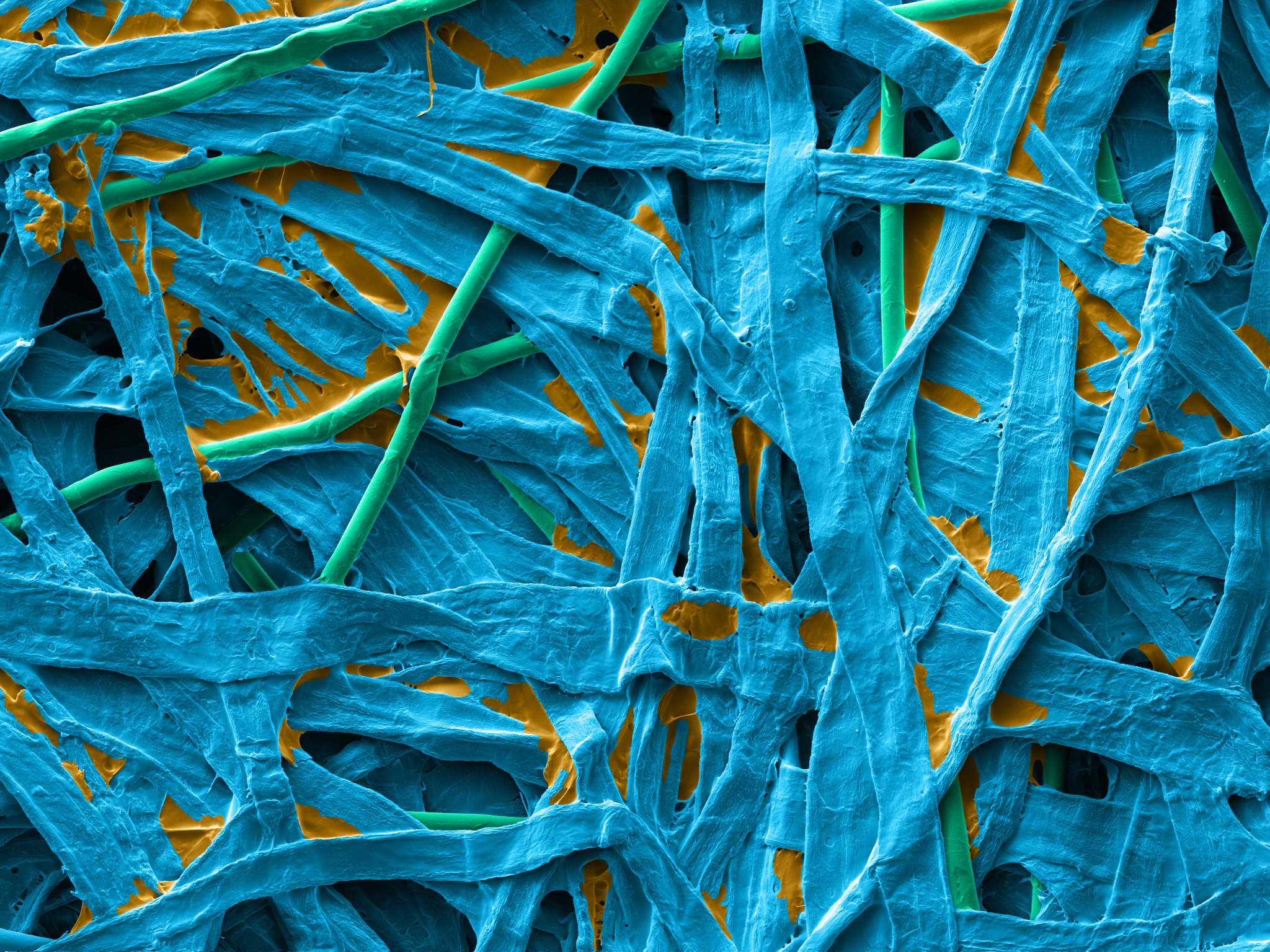
Reliance® Detex is a wetlaid nonwoven made with 70% cellulose, 20% synthetic binder and 10% PET fibers. The cellulosic fibers create the best-in-class tortuous path for excellent sterility maintenance when synthetic binders and PET fibers bring more strength and softness to the product.
 |
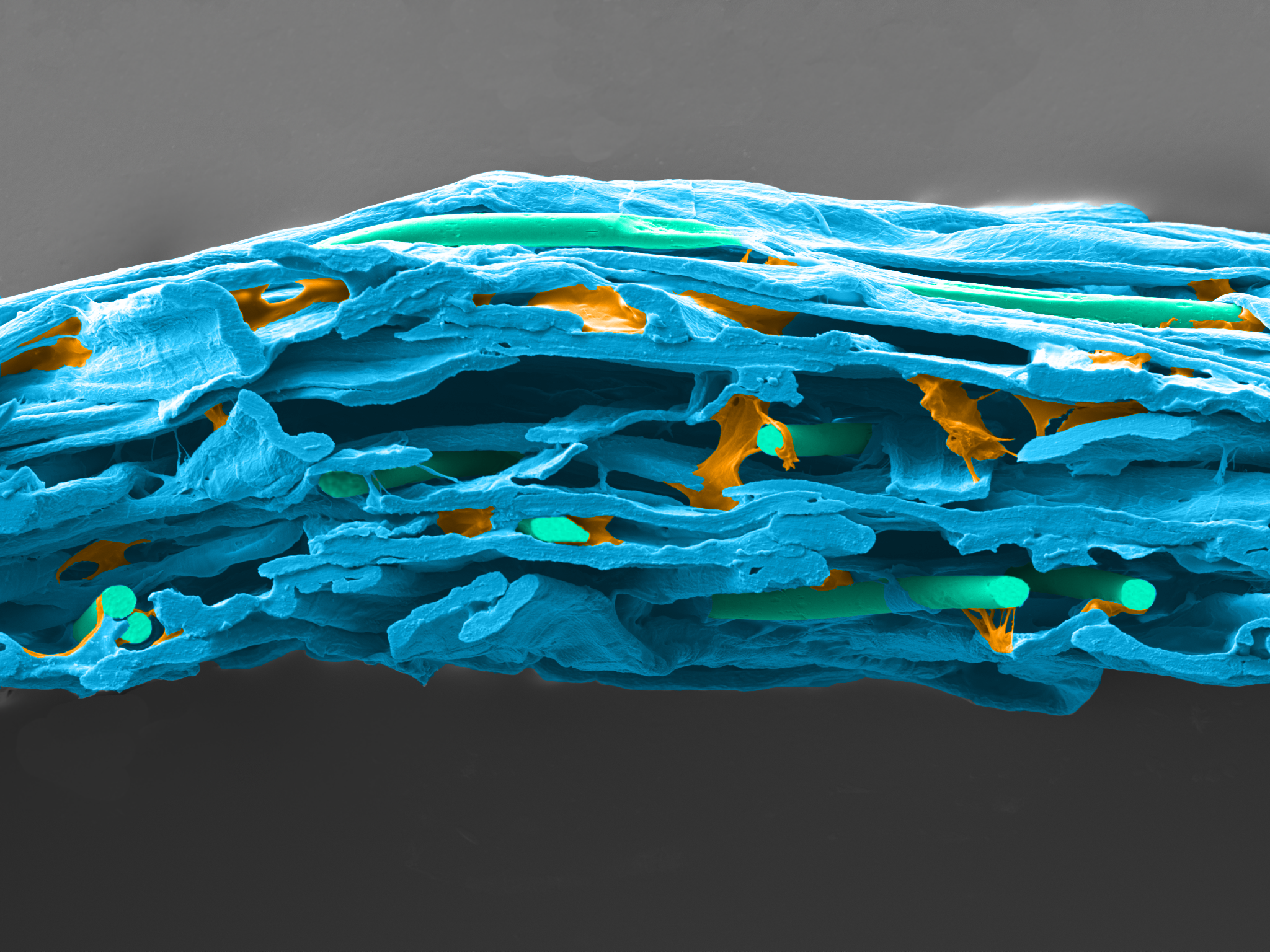 |
| Dextex wetlaid surface | Dextex wetlaid section |
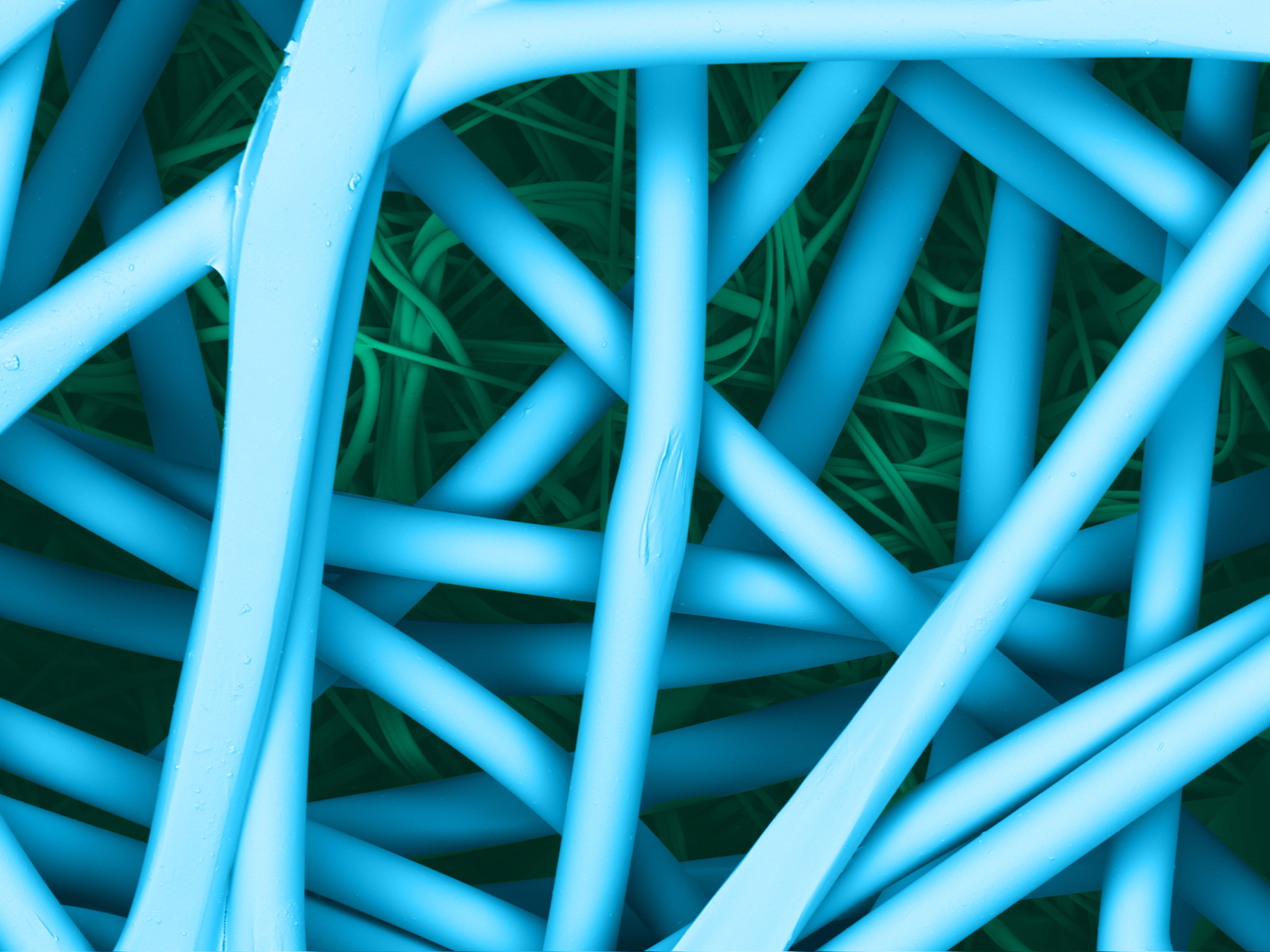
Reliance® SMS is 100% polypropylene and treated to resist static charge build-up. The key to the barrier protection of the SMS are the three meltblown layers of microscopic fibers, delivering tortuous path for a consistent bacteriological barrier to maintain sterility.
 |
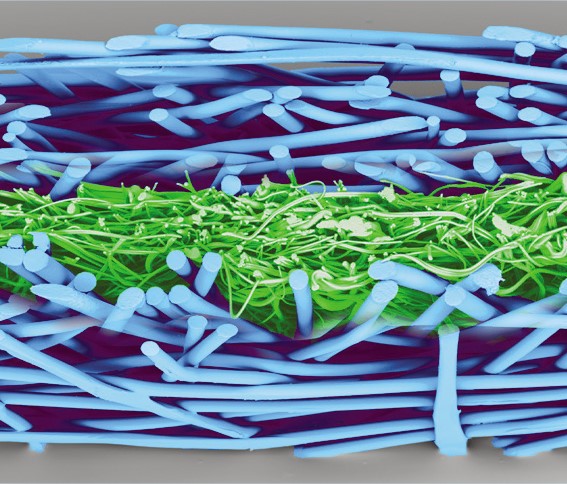 |
| SMS surface | SMS section |